Join Paul in a live webinar as he describes AS9100D, the changes from the previous revision AS9100C and what it takes to implement AS9100D in your organization. Aerospace business tends to be long term contracts, higher margins and consistent demand. AS9100D is a great way to diversify your small business product mix and strengthen your existing quality system.
According to the 2015 Aerospace Manufacturing Attractiveness Rankings Utah ranks 4th in all states for future growth in Aerospace and the rate of growth in Aerospace supply chain requirements is accelerating. If you want your company to be part of this growth you must be registered to the AS9100D (or upgrade from the AS9100C revision) quality system standard.
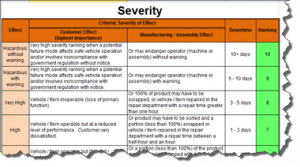
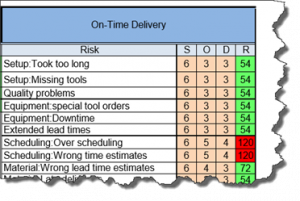
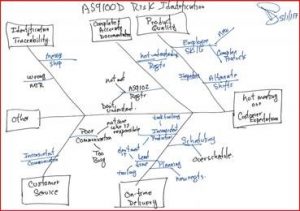
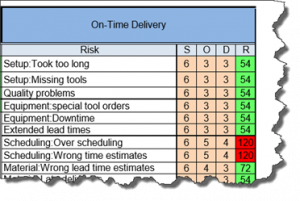
The current revision of the aerospace sector specific standard AS9100D is a “best practice” that has been developed by industry feedback to assure product quality through prevention. The standard has the flexibility to be structured differently for small organizations and large organizations, is focused on increasing customer value and is integrated with the best practice improvement philosophies like lean and 6-sigma.
To register, email info@mep.utah.edu
About Paul:
Paul Harbath is an industry expert with over 30 years of hands on experience in implementing quality management systems and helping small companies understand/implement Lean/6-Sigma. Paul has worked with both large and small organizations and has a demonstrated ability to connect with the value adding employees by simplifying complex technical issues. Paul’s company “Operational Excellence Services” specializes in small manufacturing companies.




 Paul Harbath is an industry expert with over 30 years of hands on experience in helping small manufacturers understand/implement quality management systems and lean/6-Sigma. Paul has a demonstrated ability to connect with the value adding employees by simplifying complex technical issues. Connect with him on
Paul Harbath is an industry expert with over 30 years of hands on experience in helping small manufacturers understand/implement quality management systems and lean/6-Sigma. Paul has a demonstrated ability to connect with the value adding employees by simplifying complex technical issues. Connect with him on